Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
JNC TN8400 2000-012, 33 Pages, 2000/04
The redox condition of near-field is expected to affect the performance of engineered barrier system. Especially, the oxygen initially existing in the pore space of compacted bentonites strongly affects the redox condition of the near-field. For assessing the influence of the oxygen, the transport parameters of it in the compacted bentonite and consumption process should be known. Therefore, following researches were conducted. In order to understand the diffusion of dissolved oxygen (DO) in compacted bentonite and to predict the effect of DO, the effective diffusion coefficients of DO in compacted sodium bentonite were measured by electrochemistry. As the results, the following relationship between the dry density of compacted sodium bentonite and the effective diffusion coefficient of DO in compacted sodium bentonite was derived: De=1.53 0.13
0.13 10
10 exp(-2.15
exp(-2.15 0.24
0.24 10
10 p) where De is the effective diffusion coefficient (m
p) where De is the effective diffusion coefficient (m s
s ) of DO in compacted sodium bentonite and
) of DO in compacted sodium bentonite and  is the dry density (kg m
is the dry density (kg m ) of compacted sodium bentonite. The oxygen concentration in the bentonite is expected to be controlled by oxidation of pyrite as impurity in the bentonite. In order to investigate the above idea, the rates of pyrite oxidation by DO in compacted sodium bentonite were estimated from the experimental data on pyrite-bentonite systems usig the obtained effective diffusion coefficient of DO. The results show that the averages of the rate constants of pyrite oxidation by DO in the bentonite for dry densities of 0.8, 0.9, 1.0, 1.1 and 1.2
) of compacted sodium bentonite. The oxygen concentration in the bentonite is expected to be controlled by oxidation of pyrite as impurity in the bentonite. In order to investigate the above idea, the rates of pyrite oxidation by DO in compacted sodium bentonite were estimated from the experimental data on pyrite-bentonite systems usig the obtained effective diffusion coefficient of DO. The results show that the averages of the rate constants of pyrite oxidation by DO in the bentonite for dry densities of 0.8, 0.9, 1.0, 1.1 and 1.2 10
10 kgm
kgm were 1.38
were 1.38 0.32
0.32 10
10 , 1.10
, 1.10 0.24
0.24 10
10 , 1.16
, 1.16 0.35
0.35 10
10 , 9.36
, 9.36 2.23
2.23 10
10 and 7.48
and 7.48 1.92
1.92 10
10 ms
ms , respectively. The relationship between the dry density (
, respectively. The relationship between the dry density ( ) and the rate constant (k') was expressed as follows: k'=3.94
) and the rate constant (k') was expressed as follows: k'=3.94 1.06
1.06 10
10 exp(-1.33
exp(-1.33 0.28
0.28 10
10
 ) ...
) ...
Nagase, Fumihisa; Otomo, Takashi; Tanimoto, Masataka*; Uetsuka, Hiroshi
Proceedings of the 2000 International Topical Meeting on LWR Fuel Performance (CD-ROM), 15 Pages, 2000/04
no abstracts in English
Mizuta, Shunji; ;
JNC TN9400 2000-032, 38 Pages, 2000/03
lt is necessary for feasibility study of fast reactor to evaluate the oxidation of the austenitic stainless steels in the case of using for core material in carbon dioxide gas-cooled reactor. The properties for oxidation of austenitic stainless steels in carbon dioxide were surveyed in literatures and the data were selected after evaluation of factors for oxidation in carbon dioxide. The equation of oxidation in carbon dioxide for PE16, 20Cr/25Ni/Nb, 18Cr-8Ni and JNC Cladding materials were proposed. The equation for oxidation of austenitic stainless steels were expressed as upper limit for the equation according to parabolic law. The equation for JNC cladding materials (PNC316, PNC1520, 14Cr-25Ni) was proposed based the oxidation behavior of 18Cr-8Ni which is same oxidation region for weight gain in three-component system of Fe-Cr-Ni, in addition to evaluate of effect for silicon content. The oxidation equation of 20Cr/25Ni/Nb was applied to the high Ni alloy of JNC cladding material. The obtained equation is as follows, X = 4.4W 1000, W =
1000, W =  , kp =
, kp =  exp(-Q/(RT)), X: oxide thickness[
exp(-Q/(RT)), X: oxide thickness[ m], W : weight gain[g
m], W : weight gain[g cm
cm ], kp : parabolic rate constant[g
], kp : parabolic rate constant[g
 cm
cm
 s
s ], t :time[sec]
], t :time[sec]  : constant[g
: constant[g
 cm
cm
 S
S ], Q : activation energy[J・mol
], Q : activation energy[J・mol ], R : gas constant[8.314J
], R : gas constant[8.314J  K
K
 mol
mol ], T : temperature[K] (1) PE16 : kp = 1.090
], T : temperature[K] (1) PE16 : kp = 1.090 10
10 exp(-192,500/(RD)), (2) 20Cr/25Ni/Nb : kp = 1.651
exp(-192,500/(RD)), (2) 20Cr/25Ni/Nb : kp = 1.651 10
10 exp(-201,300/(RT)) High Ni alloy (JNC), (3)18Cr-8Ni : kp = 1.503
exp(-201,300/(RT)) High Ni alloy (JNC), (3)18Cr-8Ni : kp = 1.503 10
10 exp(-60,000/(RT)), (4) PNC316, PNC1520 : kp = 1.503
exp(-60,000/(RT)), (4) PNC316, PNC1520 : kp = 1.503 10
10 exp(-60,000/(RT))
exp(-60,000/(RT)) 0.62
0.62 14Cr-25Ni(JNC) The weight gain is (3)
14Cr-25Ni(JNC) The weight gain is (3)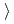 (4)
(4)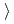 (2)
(2)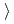 (1) in order.
(1) in order.
Nakamura, Jinichi; ; ; Kawasaki, Satoru
Journal of Nuclear Materials, 200, p.256 - 264, 1993/00
Times Cited Count:14 Percentile:78.06(Materials Science, Multidisciplinary)no abstracts in English
Shindo, Masami; Kondo, Tatsuo
JAERI-M 8835, 14 Pages, 1980/04
no abstracts in English
Shindo, Masami; Kondo, Tatsuo
JAERI-M 8770, 18 Pages, 1980/03
no abstracts in English
Shindo, Masami; ; Kondo, Tatsuo
JAERI-M 8210, 12 Pages, 1979/04
no abstracts in English
; ;
JAERI-M 6181, 14 Pages, 1975/07
no abstracts in English
Yamamoto, Masahiro; Kato, Chiaki; Motooka, Takafumi; Irisawa, Eriko; Ban, Yasutoshi; Ueno, Fumiyoshi
no journal, ,
Stainless steels used in nuclear fuel reprocessing plant occur intergranular corrosion by boiling nitric acid solution containing some cations. Reduction reaction of these cations accelerates corrosion rate of stainless steel, and then, they are re-oxidized to initial state in bulk nitric acid solution. These re-oxidized cations repeatedly concern corrosion reaction of stainless steel. The re-oxidation rates of typical cations were analyzed in the present work. As the result, Np ion accelerates corrosion of stainless steel in a little amount because it has both large reduction reaction rate and re-oxidation rate.